Electrochemical behavior of zinc layer anodes used for galvanic protection of steel in reinforced concrete
DOI:
https://doi.org/10.21809/rilemtechlett.2018.68Abstract
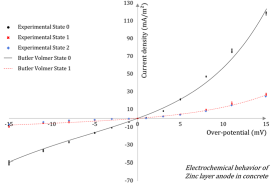
Steel corrosion is the most common reason for the premature deterioration of reinforced concrete structures. Consequently, cathodic protection of steel in concrete has been substantially developed during the past two decades. In particular, galvanic protection consists in generating a natural macrocell corrosion system in which a sacrificial metallic anode (zinc, typically) is involved to apply a cathodic polarization to the corroding steel layout, in order to mitigate or annihilate the corrosion kinetics. Whether the general principle of cathodic protection is not questionable, the global design process can be significantly improved by increasing the knowledge on electrochemical behaviours of the different components of the protecting system. Regarding zinc anodes in concrete, the literature is very scarce. The time evolution of such systems is also not rigorously addressed, aging effects are systematically ignored and zinc anodes are usually considered as non-polarizable and inert over time. In this paper, the polarization response of a zinc layer anode (ZLA) in concrete electrolyte and its time evolution are studied. The results show a rapid evolution of the ZLA behavior, once the protecting system is connected to steel reinforcements. Moreover, the characterization of ZLA provided relevant electrochemical properties for the numerical design of galvanic protection systems.
Downloads
Published
How to Cite
Issue
Section
License
Authors retain copyright of the articles published in RILEM Technical Letters and grant the journal the right of first publication with open access. The work is simultaneously licensed under Creative Commons Attribution 4.0 International License (CC BY 4.0) that allows others to share and adapt the work under the following terms: 1) a proper attribution is given in a form of a reference to the original work's authorship and initial publication in RILEM Technical Letters (bibliographic record with the DOI link); 2) a link to the license is provided; 3) the changes (if any) are indicated.









